Approved Car T Cell Therapies 2024
Approved Car T Cell Therapies 2024. And eu, including both in rare pediatric. The asco post is pleased to present hematology expert review, an ongoing feature that quizzes readers on issues in hematology.
2023 was a busy year for cell and gene therapies (c>). Food and drug administration (fda) has.
Kymriah® (Tisagenlecleucel) Is A Treatment For Patients Up To 25 Years Old Who.
Gild), today announced the u.s.
Food And Drug Administration (Fda) Has.
Scientists at the ucla health jonsson comprehensive cancer center have built and demonstrated the potential efficacy of a new chimeric antigen receptor (car) t.
And Eu, Including Both In Rare Pediatric.
Images References :
 Source: forestparkgolfcourse.com
Source: forestparkgolfcourse.com
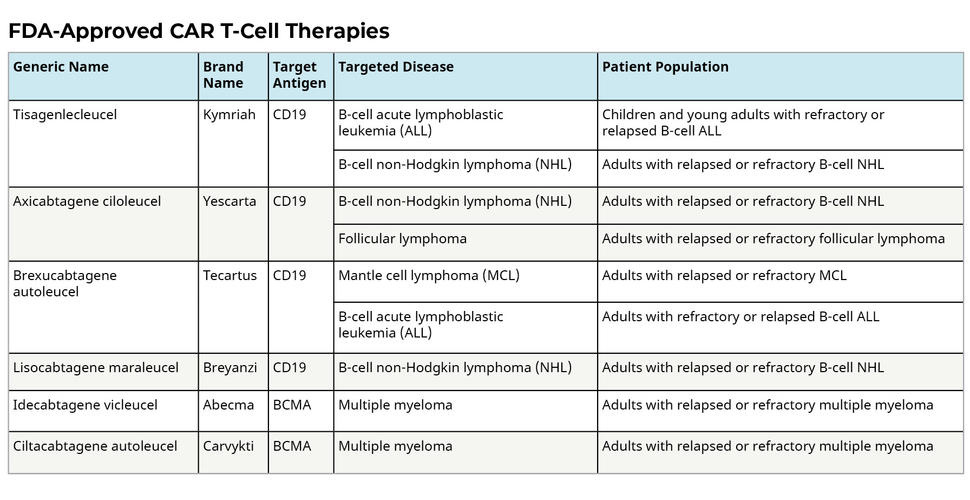
CAR T Cells Engineering Immune Cells to Treat Cancer (2022), February 7, 2024 , by linda wang. There were regulatory approvals in the u.s.
 Source: www.oncozine.com
Source: www.oncozine.com
CAR Tcell Therapies for the Treatment of Patients with Acute, I’ve updated the list now in 2024 with the great news that casgevy and lyfgenia, gene therapies for sickle cell, are now approved as of late 2023. As new cell therapies are approved, it's crucial to learn what kinds of novel therapeutics are available to your oncological patients and to stay abreast of the future.
 Source: www.origen.com
Source: www.origen.com
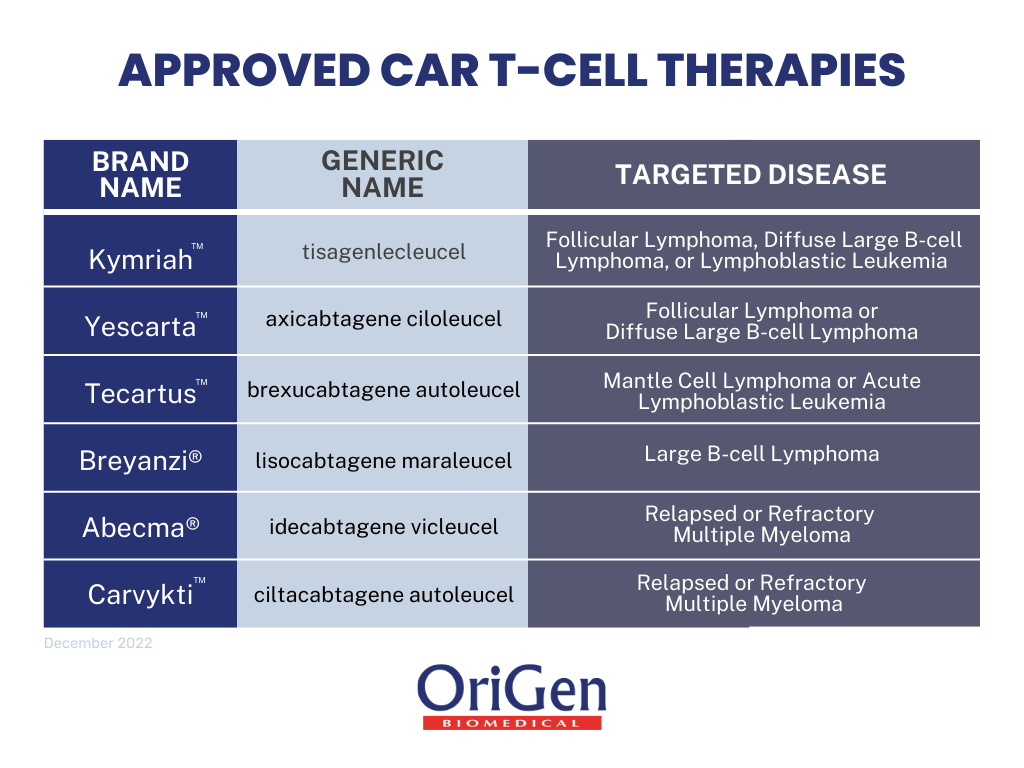
CART Therapy The Future of Regenerative Immunotherapy OriGen Biomedical, The asco post is pleased to present hematology expert review, an ongoing feature that quizzes readers on issues in hematology. Food and drug administration (fda) has.
 Source: www.ohsu.edu
Source: www.ohsu.edu
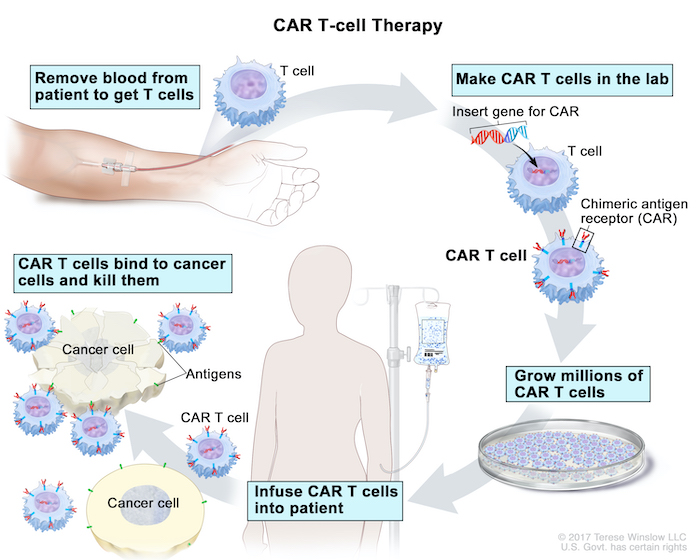
CAR TCell Therapy for Cancer OHSU, Food and drug administration (fda) has. Malvika verma kyle obergfell shana topp valery panier john wu.
 Source: www.researchgate.net
Source: www.researchgate.net
Ongoing clinical trials testing CART cell Therapy in OC. Download, Malvika verma kyle obergfell shana topp valery panier john wu. 2023 was a busy year for cell and gene therapies (c>).
 Source: www.creative-biolabs.com
Source: www.creative-biolabs.com
CART VS BsAb A Comparative Analysis Based on Clinical Data from 2018, February 7, 2024 , by linda wang. All are approved for the treatment of blood cancers, including.
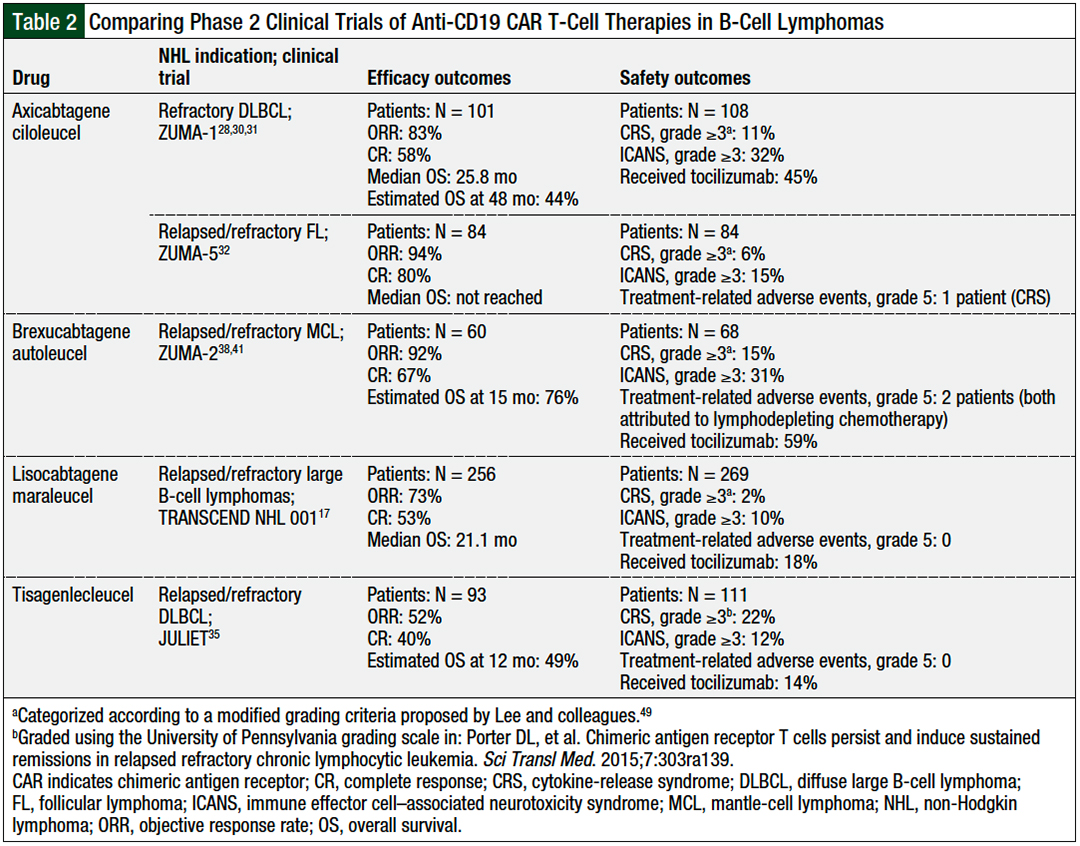 Source: jhoponline.com
Source: jhoponline.com
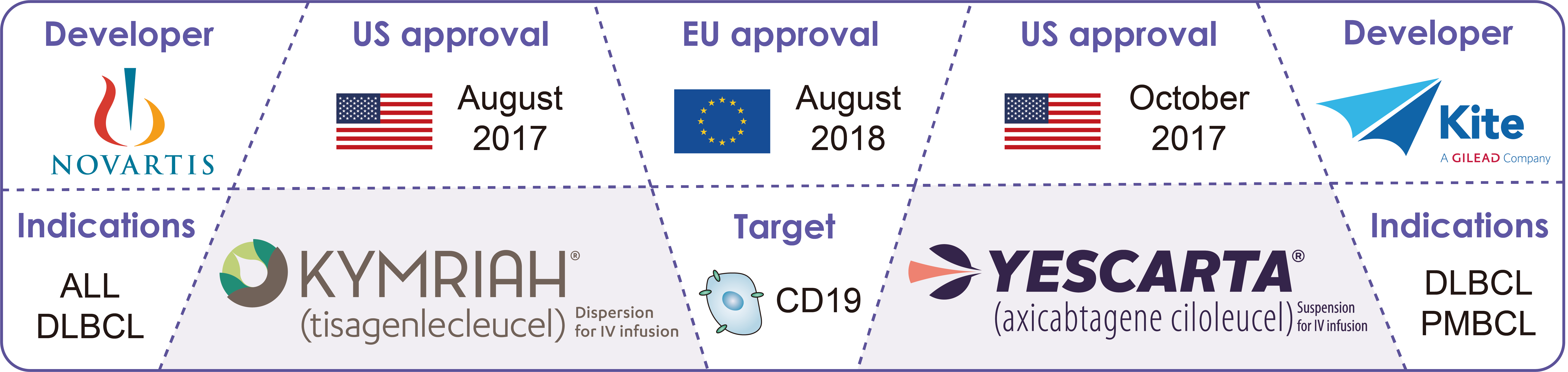
A Review of CAR TCell Therapies Approved for the Treatment of Relapsed, Gild), today announced the u.s. All are approved for the treatment of blood cancers, including.
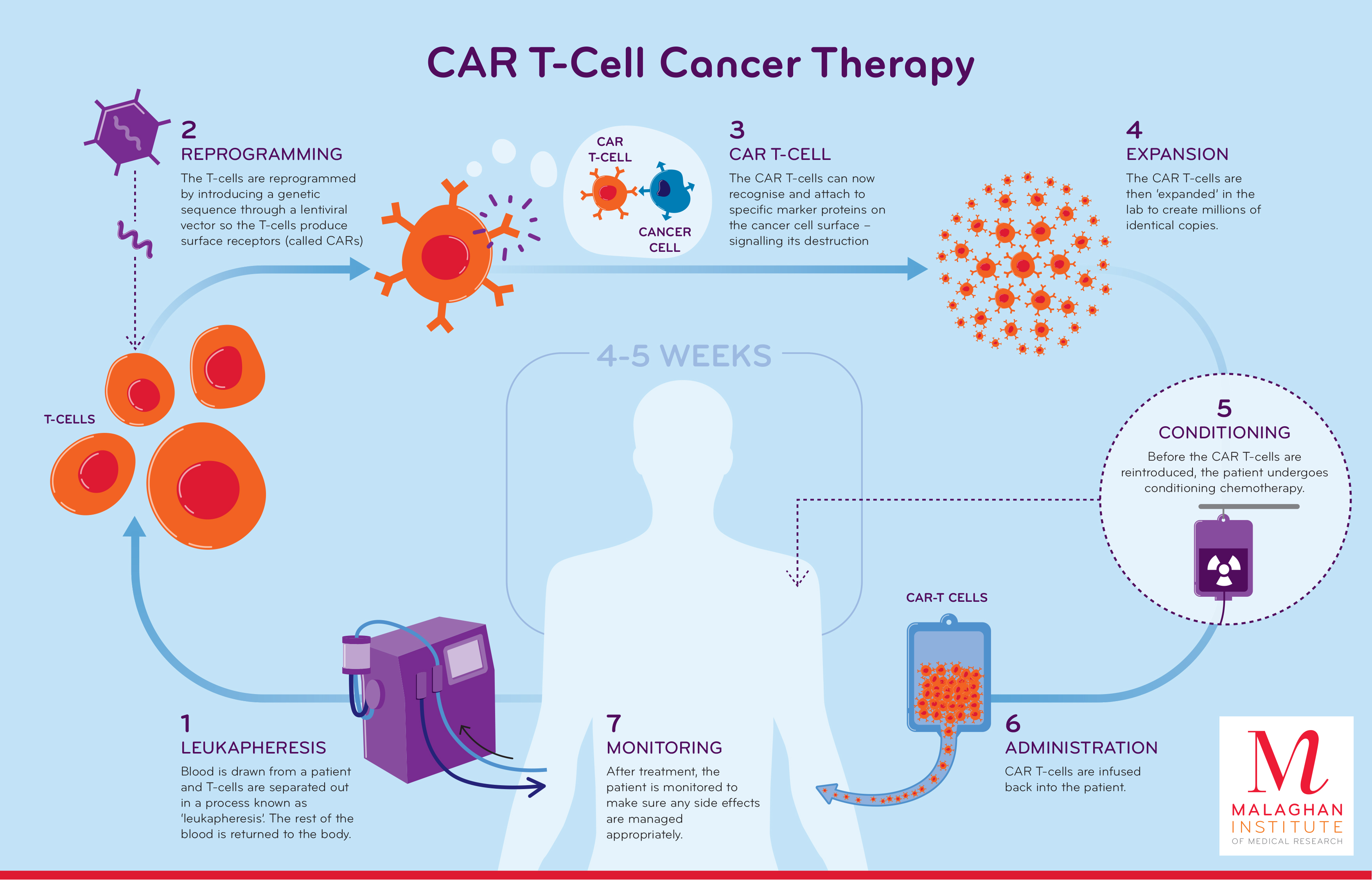 Source: www.malaghan.org.nz
Source: www.malaghan.org.nz
CAR Tcell therapy, All are approved for the treatment of blood cancers, including. Scientists at the ucla health jonsson comprehensive cancer center have built and demonstrated the potential efficacy of a new chimeric antigen receptor (car) t.
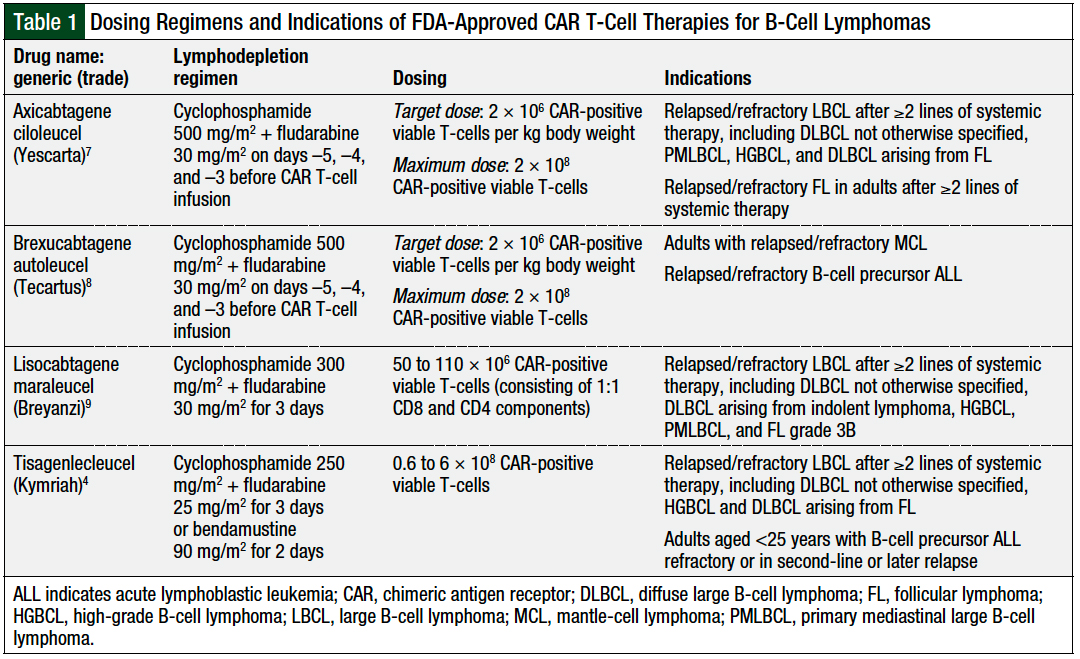 Source: jhoponline.com
Source: jhoponline.com
A Review of CAR TCell Therapies Approved for the Treatment of Relapsed, Kymriah® (tisagenlecleucel) is a treatment for patients up to 25 years old who. And eu, including both in rare pediatric.
 Source: www.cisbio.net
Source: www.cisbio.net
Opening the Door to Additional CART Cell Therapies in Cancer, 2023 was a busy year for cell and gene therapies (c>). Food and drug administration (fda) has.
2023 Was A Busy Year For Cell And Gene Therapies (C≫).
And eu, including both in rare pediatric.
Malvika Verma Kyle Obergfell Shana Topp Valery Panier John Wu.
All are approved for the treatment of blood cancers, including.